Acid Base Equilibrium Practice Test
Practice Test 161 pg 1 of 3 Acid Base Equilibrium The Ka of hydrazoic acid HN3 is 19 x 10-5 at 250 C. Kab -14for a conjugate acidbase pair Quadratic Equation.

Acid Base Questions Practice Khan Academy
Which statement about buffer systems is not correct.
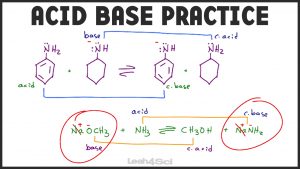
. The state of equilibrium can be maintained over time as long as. Acid Base Practice Problems Master Organic Chemistry Acid Base - Question 1. Acids Bases and Conjugates Miscellaneous 1.
The buffer region is marked by the letter __. This free online practice. Ap Chemistry Acid Base Equilibrium Practice Test and Practice Exam are the textbook and this website is the perfect place to practice for the AP Chemistry exam.
This online quiz is intended to give you extra practice in calculations involving weak acids and bases including K a K b pH and ion and solution concentrations. In the BrønstedLowry definition of acids and bases an acid _____ a. A Its a dynamic equilibrium.
Electron rich species are called lewis base. A buffer system may contain. Detailed Solution for Test.
Up to 24 cash back RTD Chemistry 30 Unit 2 Equilibrium Focussing on Acids and Bases 7 NR 5 Weak acid-strong base titration curve. Under the premise of a chemical equilibrium which of the following can be presumed. A buffer system may contain a weak acid and its conjugate base.
Whose definition of acids and bases emphasizes the role of protons. Download my free guide 10 Secrets to A. Practice calculations involving solutions of weak acids and bases in this set of free practice questions designed for AP Chemistry students.
Strong bases are a. SCH 4U Acid Base Equilibrium Practice Test Answers can be found on my website 1 aq. A Bronsted-Lowry acid is a proton donor and a Bronsted-Lowry.
In the BrønstedLowry definition of acids and bases an acid _____ a. A buffer system may contain a weak base and its conjugate. X a bbac 2 24 1.
A buffer system may contain a weak base and its conjugate. - Lets look at two definitions for acids and bases Bronsted-Lowry and Lewis and well start with Bronsted-Lowry. Is a proton donor.
What is the pH of a 035 M aqueous solution of HN3. Organic Chemistry Acid Base Equilibrium Practice QuestionsNeed help with Orgo. Which of the following is false about a system at equilibrium.
View acid_test_practicedoc from CHEM 115 at Mount Royal University. A model of acid-base behavior based on proton transfer in which an acid and a base are defined respectively as a species that donates a proton and one. If an acid is combined with a base of equal strength the result will most likely be.
Practice Test 161 pg 1 of 3 Acid Base Equilibrium The Ka of hydrazoic acid HN3 is 19 x 10-5 at 250 C. Among the given BF 3 is an electron deficient species so. None of these answers are correct.
Acid Base - Question 1. Impossible to tell without testing the pH.

Acid Base Equilibrium Organic Chemistry Practice Questions
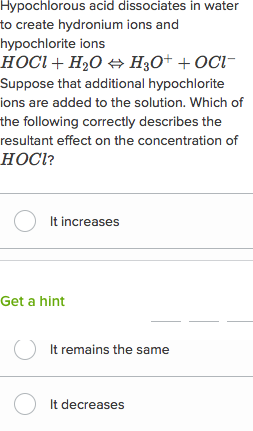
Acid Base Questions Practice Khan Academy

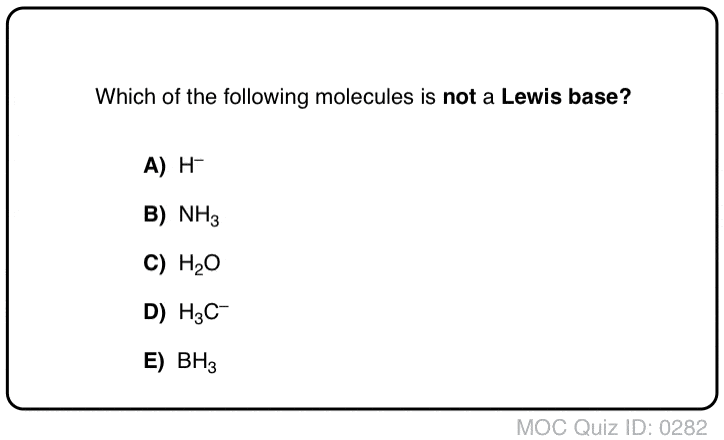
No comments for "Acid Base Equilibrium Practice Test"
Post a Comment